Different Experimental Error Chemistry Student Faces During Titration
Asked By
10 points
N/A
Posted on - 11/03/2017

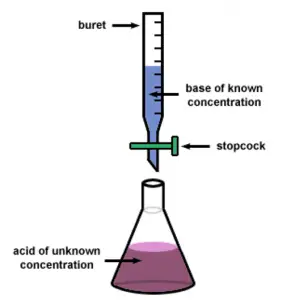
I am doing chemical titration for the very first time. What are the different experimental error chemistry student faces during titration? Reply ASAP.